Read the Letter: International Human Right to Informed Consent Prior to Medical Procedures and Vaccination Choice
View the World Medical Association Medical Ethics Manual
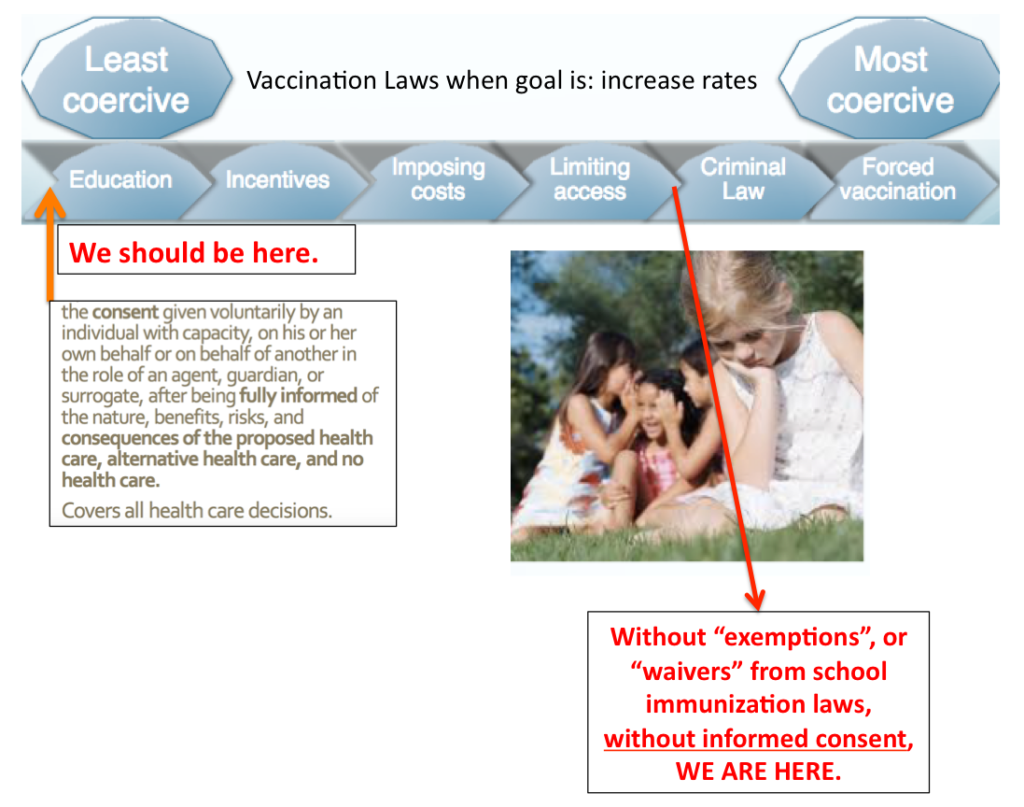
Mandatory health, medical and vaccination policies violate informed consent. Incentivizing medical procedures is a form of medical coercion.
Nuremburg Code
The Nuremburg Code, established in 1948, was developed from the Trials of War Criminals before the Nuremberg Military Tribunals. It defines ethical behavior in the conduct of research using human subjects. Its development grew from the atrocities in human research conducted in Nazi Germany. The Nuremberg Code was the first international document that advocated voluntary participation and informed consent.
Declaration of Helsinki
In 1964, the World Medical Association (WMA) developed the as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving humans subjects, including research on identifiable human material and data. The code of ethics has been widely adopted by medical associations in various countries. It is regularly revised and amended by the WMA, most recently in October 2013, and is the basis for Good Clinical Practices used today.
National Research Act
The National Research Act was passed by Congress in 1974 after the unethical U.S. Public Health Service Syphilis Study at Tuskegee ended. The Act established the existence of IRBs to review biomedical and behavioral research involving human subjects. It also created the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Commission was tasked in identifying the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects, and developing guidelines to assure that such research is conducted in accordance with those principles.
American Medical Association Code of Ethics (2018)

Title 18 : Health, Chapter 231 : Advance Directives For Health Care, Disposition Of Remains, And Surrogate Decision Making (18 V.S.A. § 9701) defines informed consent as: the consent given voluntarily by an individual with capacity, or his or her own behalf or another in the role of an agent, guardian, surrogate after being fully informed of the nature, benefits, risks and consequences of the proposed health care, alternative health care, and no health care.
This should apply to all decisions – even in times of emergency and especially for vaccinations: Consent means having the right to say no.
We seek to bolster and improve protections for both patients and doctors, guaranteeing fully informed consent prior to vaccination. This will build trust in the doctor-patient relationship and honor the freedom and rights of patients and parents to make autonomous medical decisions for themselves and their family members.
H310, introduced in 2018/2019 failed to get a hearing, but would have make it medical malpractice for failure warn vaccine consumers of the risks associated with a vaccine product.
Doctors and other healthcare providers report feeling pressured into forcing vaccines when really they should be abiding by the doctrine of informed consent.
Consumers report being coerced.
Ethical medicine does not coerce a person to undergo the procedure.
This bill would have ensured that informed consent is followed, even in case of public health measures/emergencies.
“Informed Consent Prior to Immunization” should mean: consent given voluntarily by an individual with capacity, or his or her own behalf or another in the role of an agent, guardian, surrogate after being fully informed of the nature, benefits, risks and consequences of the proposed vaccine, alternative vaccine, and no vaccine. You see – vaccines are given to healthy people. We ask that the State public health programs be at least:
- Providing list of ingredients to patient or parent
- Reviewing safety concerns
- Letting the patient know the procedure is voluntary and documenting this
- Documenting having received the written consent of adult, or parent (if minor is under age 18)
- Avoiding coercion
* In Vermont, the age of consent for medical procedures (including vaccinations) is eighteen (18) years old (see page 24 here).
CLICK FOR TALKING POINTS file
Formal Informed Consent prior to any vaccination is needed because:
1. Vaccines are not given for treatment of any disease; these are healthy children with caring parents making medical decisions for their own.
2. Vaccines, acknowledged to be “unavoidably unsafe”, are given to HEALTHY children and adults, and thus their administration requires the higher ethical bar of formal informed consent ( informed consent as set forth in the 1948 Nuremberg Code, the 2005 UNESCO Declaration, and the 2018 AMA code of Ethics – which is adopted by most state medical societies, including Vermont’s ).
3. Public health agencies, health care personnel, and drug makers bear no liability in the event that a vaccine causes serious disability or death.
4. Vaccines are classified as “biologics” and thus are exempt from the rigorous safety screening and scrutiny that is required for prescription drugs.
Consumers should be given adequate information in the form of the manufacturer vaccine package insert, with adequate time to read it and have questions answered at the vaccine appointment. The provider will need to keep a record of written informed consent (patient or parent of minor under age 18).
But, to quote T. Leonardo Alves (who wrote in Science and Engineering Ethics in May 2018): “… any decision to use a medicine involves weighing potential benefits against possible harms. To make an informed decision, a person needs information on the aims of the treatment, how it works, how to use it properly, the likelihood of benefit and harm, and how this medicine compares with other available treatment options or the option not to treat, as well as relative cost-effectiveness. The quality of information that accompanies medicines can make the difference between ‘a poison and a cure’, between a use that leads to better health and a use most likely to lead to harm. Of equal importance to information on medicines, inaccurate information on diseases and disease risks can lead to harm if patients seek medical treatment when it is not needed, leading to unnecessary medicine use and the potential exposure to drug-induced harm.”
And, to quote Jun Ma, MD PhD of the Stanford Center for Research in Disease Prevention: “Knowing that drug promotion is done primarily for marketing — not education — physicians and the public need to analyze this information more objectively and be more proactive in seeking out information about the products being promoted.”
Informed choice is the only way forward.
Doctors should not coerce – they should warn and inform.
The ingredients, warnings and precautions that Merck, Sanofi, Glaxo, Pfizer and other pharmaceutical companies give to pediatricians about their vaccine products should be conveyed to consumers (parents) – but this is currently not required by law in Vermont. Time for a change!